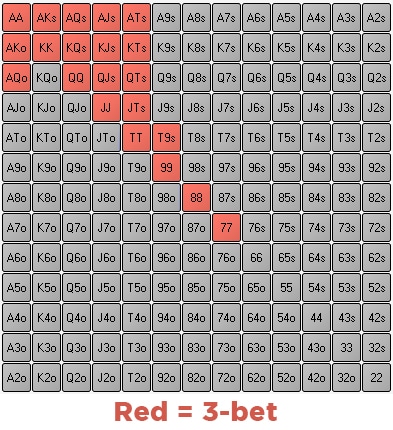
What Is A Three Bet
Increase your preflop raise size when there is a weak player in the blinds. The main goal for this. The standard (and often implied) number is –110, meaning a successful bet of $110 would net $100 profit. This is the “juice” or “vigorish” (aka “vig”) for the house or sportsbook. Whereas a typical moneyline bet involves a bet on one of two options, three-way moneyline betting involves three options. When wagering on a three-way moneyline, you can bet either Team A to win, Team B to win, or for the event to end in a tie (sometimes referred to as a ‘draw’).
Overview of BET Theory
The BET theory was developed by Stephen Brunauer (Figure (PageIndex{1}) ), Paul Emmett (Figure (PageIndex{2}) ), and Edward Teller (Figure (PageIndex{3}) ) in 1938. The first letter of each publisher’s surname was taken to name this theory. The BET theory was an extension of the Langmuir theory, developed by Irving Langmuir (Figure (PageIndex{4}) ) in 1916.
The Langmuir theory relates the monolayer adsorption of gas molecules (Figure (PageIndex{5}) ), also called adsorbates, onto a solid surface to the gas pressure of a medium above the solid surface at a fixed temperature to ref{1} , where θ is the fractional cover of the surface, P is the gas pressure and α is a constant.

[ Theta = frac{alpha cdot P}{1 + (alpha cdot P)} label{1} ]
The Langmuir theory is based on the following assumptions:
- All surface sites have the same adsorption energy for the adsorbate, which is usually argon, krypton or nitrogen gas. The surface site is defined as the area on the sample where one molecule can adsorb onto.
- Adsorption of the solvent at one site occurs independently of adsorption at neighboring sites.
- Activity of adsorbate is directly proportional to its concentration.
- Adsorbates form a monolayer.
- Each active site can be occupied only by one particle.
The Langmuir theory has a few flaws that are addressed by the BET theory. The BET theory extends the Langmuir theory to multilayer adsorption (Figure (PageIndex{1}) ) with three additional assumptions:
- Gas molecules will physically adsorb on a solid in layers infinitely.
- The different adsorption layers do not interact.
- The theory can be applied to each layer.
How does BET Work?
Adsorption is defined as the adhesion of atoms or molecules of gas to a surface. It should be noted that adsorption is not confused with absorption, in which a fluid permeates a liquid or solid. The amount of gas adsorbed depends on the exposed surface are but also on the temperature, gas pressure and strength of interaction between the gas and solid. In BET surface area analysis, nitrogen is usually used because of its availability in high purity and its strong interaction with most solids. Because the interaction between gaseous and solid phases is usually weak, the surface is cooled using liquid N2 to obtain detectable amounts of adsorption. Known amounts of nitrogen gas are then released stepwise into the sample cell. Relative pressures less than atmospheric pressure is achieved by creating conditions of partial vacuum. After the saturation pressure, no more adsorption occurs regardless of any further increase in pressure. Highly precise and accurate pressure transducers monitor the pressure changes due to the adsorption process. After the adsorption layers are formed, the sample is removed from the nitrogen atmosphere and heated to cause the adsorbed nitrogen to be released from the material and quantified. The data collected is displayed in the form of a BET isotherm, which plots the amount of gas adsorbed as a function of the relative pressure. There are five types of adsorption isotherms possible.
Type I Isotherm
3 Bet Poker Meaning
Type I is a pseudo-Langmuir isotherm because it depicts monolayer adsorption (Figure (PageIndex{6}) ). A type I isotherm is obtained when P/Po < 1 and c > 1 in the BET equation, where P/Po is the partial pressure value and c is the BET constant, which is related to the adsorption energy of the first monolayer and varies from solid to solid. The characterization of microporous materials, those with pore diameters less than 2 nm, gives this type of isotherm.
Type II Isotherm
A type II isotherm (Figure (PageIndex{7}) ) is very different than the Langmuir model. The flatter region in the middle represents the formation of a monolayer. A type II isotherm is obtained when c > 1 in the BET equation. This is the most common isotherm obtained when using the BET technique. At very low pressures, the micropores fill with nitrogen gas. At the knee, monolayer formation is beginning and multilayer formation occurs at medium pressure. At the higher pressures, capillary condensation occurs.
Type III Isotherm
Texas Holdem 3 Bet
A type III isotherm (Figure (PageIndex{8}) ) is obtained when the c < 1 and shows the formation of a multilayer. Because there is no asymptote in the curve, no monolayer is formed and BET is not applicable.
Type IV Isotherm

Type IV isotherms (Figure (PageIndex{9}) ) occur when capillary condensation occurs. Gases condense in the tiny capillary pores of the solid at pressures below the saturation pressure of the gas. At the lower pressure regions, it shows the formation of a monolayer followed by a formation of multilayers. BET surface area characterization of mesoporous materials, which are materials with pore diameters between 2 - 50 nm, gives this type of isotherm.
What Is A 3-bet In Poker
Type V Isotherm
What Is A Three Bet In Poker
Type V isotherms (Figure (PageIndex{10}) ) are very similar to type IV isotherms and are not applicable to BET.